The Third Law of Thermodynamics Describes the Entropy of a
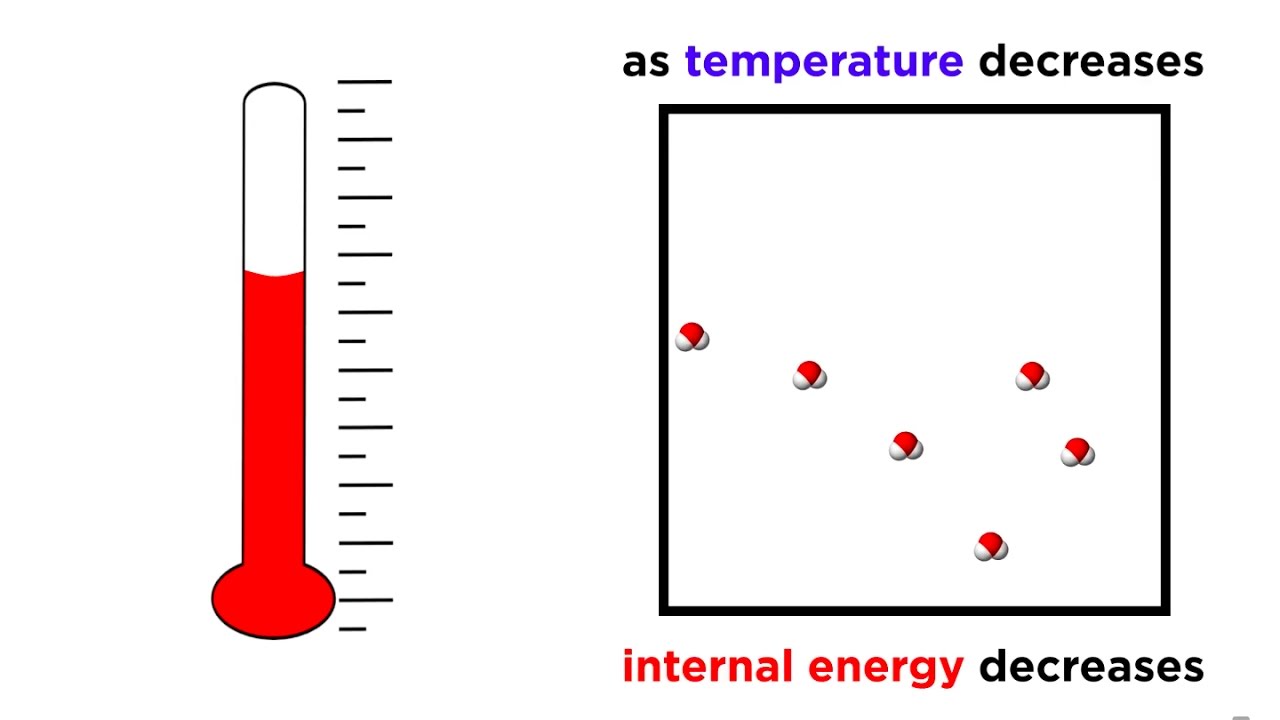
The third law of thermodynamics sometimes called Nernsts theorem or Nernsts Postulate relates to the entropy and temperature of a physical system. At zero kelvin the system must be in a state with the minimum possible energy thus this statement of the third law holds true if the perfect crystal has only one minimum energy state.

Solved Question 7 Which Of The Following Describes The Third Chegg Com
A description of any thermodynamic system employs the four laws of thermodynamics that form an axiomatic basis.

. By Jim Lucas Contributions from Ashley Hamer published 2 February 22. The third law of thermodynamics is. Solid liquid gas all of the above.
The entropy of a system approaches a constant value when its temperature approaches absolute zero. Entropy is the measure of the disorder in a system and while a perfect crystal is by definition perfectly ordered so that the entropy of that crystal is zero. 1423 The Third Law of Thermodynamics You Cannot Get Out of the Game The third law of thermodynamics states that the entropy of a system approaches a constant value as the temperature approaches absolute zero.
Expert-verified answer 50 5 7 RomeliaThurston. -The enthalpy of the universe is constant. Chemistry questions and answers.
The third law of thermodynamics says that the entropy of a perfect crystal at absolute zero is exactly equal to zero. Since entropy qt and since q0 in pure crystalline substances then entropy is 0. For a chemical reaction if the value of Gibbs free energy if zero it means that the reaction does not react.
Solid liquid gas all of the above. The third law of thermodynamics states regarding the properties of closed systems in thermodynamic equilibrium. The first law specifies that energy can be transferred between physical systems as heat as work and with transfer of matter.
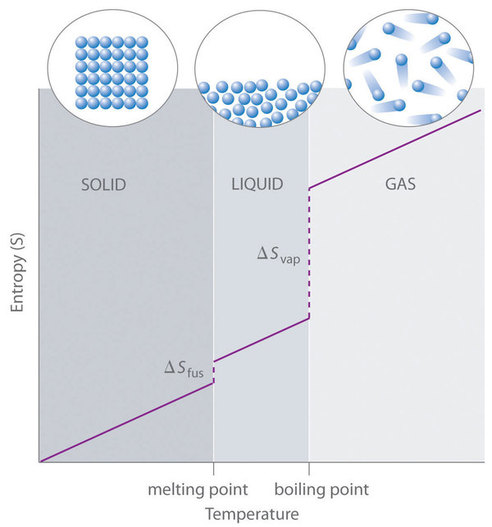
The third law of thermodynamics is sometimes stated as follows. The third law of thermodynamics describes the entropy of a. The crystal must be perfect or else there will be some inherent disorder.
If Δ Suniv 0 the process is nonspontaneous and if Δ Suniv 0 the system is at equilibrium. Third Law of Thermodynamics 3 points 1. A gas from a container decreases the entropy as it undergoes diffusion or effusion.
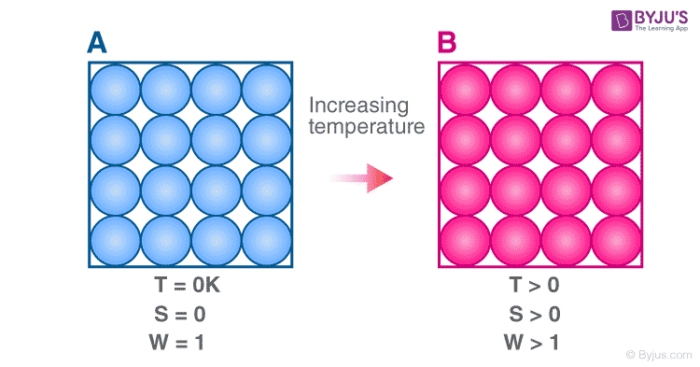
The entropy of a system at absolute zero is typically zero and in all cases is determined only by the number of different ground states it has. This constant value cannot depend on any other parameters characterizing the closed system such as pressure or applied magnetic field. -The entropy of the universe is increasing.
The second law of thermodynamics states that a spontaneous process increases the entropy of the universe Suniv 0. Third Law of Thermodynamics The third law of thermodynamics states that the entropy of a system approaches a constant value as the temperature approaches zero. The second law defines the existence of a quantity called entropy that describes the direction.
Pure crystalline substances have heat capacity of zero and cant absorbe any heat. D The energy of the universe is constant E The entropy of all elements are. Which of the following statements describes the third law of thermodynamics.
The third law of thermodynamics is concerned about the entropy of the system. The third law of thermodynamics states that the entropy of a system approaches a constant value as the temperature approaches zero. The entropy of a perfect crystal is zero when the temperature of the crystal is equal to absolute zero 0 K.
What exactly is entropy. According to the third law of thermodynamics the entropy of a perfect crystal is zero when the temperature of the crystal is equal to absolute zero 0 kelvin. Get 1-on-1 help from an expert tutor now.
Select the correct answer below. It is because at absolute zero temperature a system is in a ground state and entropy increases are achieved. The Third Law of Thermodynamics The third law of thermodynamics defines absolute zero on the entropy scale.
-The enthalpy of the universe is decreasing. The 3rd law of thermodynamics explains entropy. -The enthalpy of the universe is increasing.
If the ground state is non-degenerate then the entropy approaches zero. The entropy of a perfect crystal at absolute zero is exactly equal to zero. The third law of thermodynamics states that the entropy of a pure crystal at absolute zero is zero.
The third law of thermodynamics establishes the zero for entropy as that of a perfect pure crystalline solid at 0 K. This states that the entropy of the perfect crystal is zero only when the temperature of the crystal is equal to the absolute zero which is equal to zero kelvin. Select the correct answer below.
But hold on a minute. -The entropy of a perfect crystal is zero at 0 K. It is the hypothetical temperature at which all molecular motion stops.
Well entropy is a measure of. Pure Crystlalline Substances occur at absolute zero temperature. The entropy of a system at absolute zero is typically zero and in all cases is determined only by the number of different ground states it has.
This principle states that the entropy of a system at absolute zero temperature is a well-defined constant. A The entropy B The entropy of the universe is constant. Absolute zero The coldest temperature 0 Kelvin that can be reached.
The 3rd law of thermodynamics states that as the temperature of a system approaches zero then the entropy of the system approaches zero or some positive constant. The third law of thermodynamics describes the entropy of a. -The entropy of the universe is decreasing.
According the second law of thermodynamics if the system is randomly arranged it has much higher entropy than that of the properly arrange. C The entropy at T-0K is zero.
The Third Law Of Thermodynamics Absolute Zero Youtube
13 6 The Third Law Of Thermodynamics Chemistry Libretexts
Third Law Of Thermodynamics Entropy At Absolute Zero

The Third Law Of Thermodynamics Its Application To Absolute Entropy Video Lesson Transcript Study Com

Pdf The Third Law Of Thermodynamics Or An Absolute Definition For Entropy Part 1 The Origin And Applications In Thermodynamics

4 Laws Of Thermodynamics With Examples Very Simple

4 Laws Of Thermodynamics With Examples Very Simple
0 Response to "The Third Law of Thermodynamics Describes the Entropy of a"
Post a Comment